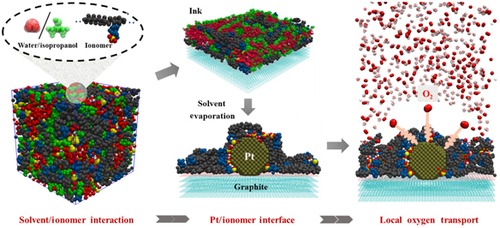
Molecular dynamic simulations are employed to investigate the interaction between the ionomers and solvents in catalyst ink, and the corresponding structure and oxygen transport property of Pt/ionomer interface, which crucially impacts the performance of low-Pt proton exchange membrane fuel cells (PEMFCs). When dispersed in solvent dominated with isopropanol which has a relatively low dielectric constant, the perfluorosulfonic acid (PFSA) ionomers form reverse micelle-like structures constituted of clusters of compact sulfonate-hydronium ion pairs encircled by backbone chains, which sustain when forming catalyst ink and inhibit the adsorption sulfonate groups on Pt, and consequently result in relatively low-density but thick ionomer assemblies around Pt in catalyst layer (CL). The strong solvation and dielectric screening ability of water tend to make the sulfonate group evenly distribute in solution, thus facilitating the sulfonate adsorption and inducing dense ionomer films on Pt in CL. Therefore, the ionomer films tend to become thinner yet more compact around Pt with the addition of water into the isopropanol-based catalyst ink, resulting in a shortened oxygen transport path but decreased oxygen diffusivity. Accordingly, there is a non-monotonic relationship between oxygen transport resistance and water content in the mixing solvents, and optimization is achieved at a water volume fraction of 50 vol%.
https://doi.org/10.31635/renewables.024.202400059