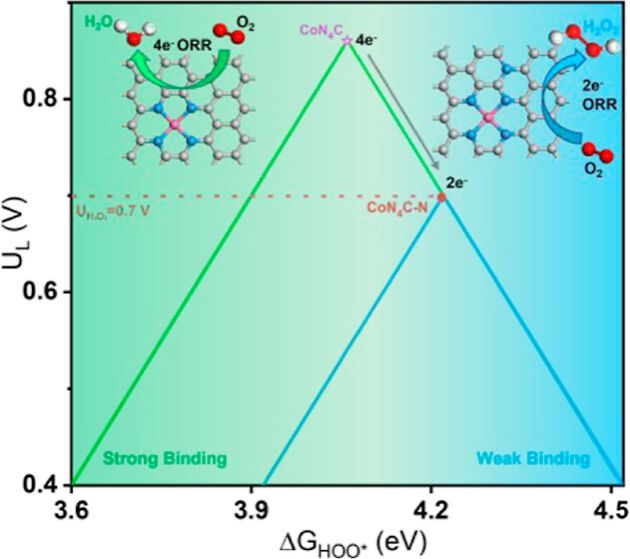
The catalytic performance of single-atom catalysts (SACs) is vitally determined by their coordination environments. So far, the catalytic manipulation of SACs has been mainly focused on the first and second nearest coordination structures of the center atoms. We herein demonstrate that the chemical environments beyond the second coordination shells also significantly influence the catalytic behaviors of SACs. Our findings reveal that the presence of graphitic nitrogen can induce a shift of the O2 reduction pathway on CoN4C sites from an energy-conversion favorite 4e– pathway to a H2O2-production desirable 2e– pathway. The remote graphitic N tunes the electronic structure of Co from the lower-spin state to the higher-spin state, as proved by the zero-field cooling (ZFC) temperature-dependent magnetic susceptibility, which weakens the adsorption of O2/*OOH, ultimately enhancing the selectivity toward H2O2. It is further revealed that the catalytic influence of graphitic N may be universally present in other SACs such as FeN4C and MnN4C. Impressively, the graphitic N-doped CoN4C exhibits a high H2O2 Faraday efficiency (82%) in a flow cell, with a remarkable H2O2 yield of 0.096 mmol cm–2 h–1 for 200 h at 0.358 V (vs RHE), which is sufficient for many applications such as the electro-Fenton-like degradation of the malachite green and demonstrated the feasibility of H2O2 electrosynthesis.
https://pubs.acs.org/doi/10.1021/acscatal.4c00781