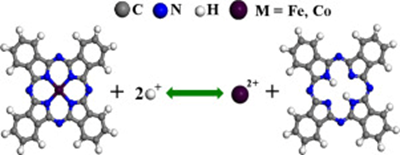
Fe and Co porphyrins and phthalocyanines are excellent catalysts for the oxygen reduction reaction (ORR) and are promising alternatives to Pt in fuel cells. However, the stability of these molecular catalysts in acidic media is poor. This study explores whether demetalation through proton ex‐ change causes these metal macrocyclic catalysts to be unstable in acidic media. We first present a theoretical scheme for investigating exchange reactions of metal ions in metal macrocyclic com‐ pounds with protons in acidic media. The equilibrium concentrations of metal ions in solution when various metalloporphyrins (MPs) and metallophthalocyanines (MPcs) are brought into contact with a strongly acidic solution (pH = 1) were then estimated using density functional theory calculations; these values were used to evaluate the stability of these metal macrocyclic compounds against demetalation in acidic media. The results show that Fe, Co, Ni, and Cu phthalocyanines and porphy‐ rins have considerable resistance to exchange with protons, whereas Cr, Mn, and Zn phthalocya‐ nines and porphyrins easily undergo demetalation through ion exchange with protons. This sug‐ gests that the degradation in the ORR activity of Fe and Co macrocyclic molecular catalysts and of carbon materials doped with Fe(Co) and nitrogen, which are believed to have metal‐nitrogen coor‐ dination structures similar to those of macrocyclic molecules as ORR catalytic centers, is not the result of replacement of metal ions by protons. The calculation results show that electron‐donating substituents could enhance the stability of Fe and Co phthalocyanines.
https://doi.org/10.1016/S1872-2067(15)61082-8