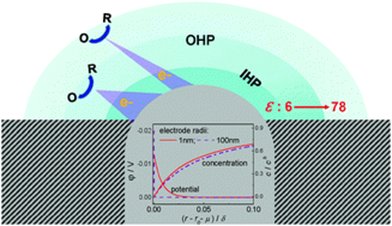
Electrodes of nanometer sizes provide a model approach to study the nanoscale electrochemical properties and processes, which are of fundamental and applied significance in a variety of areas including energy and environmental science, scanning probe microscopies, nanofabrication as well as electrochemistry itself. This Perspective reviews recent developments in conceptual understanding, theoretical modelling and simulation, and experimental observation of nanosize-induced properties and phenomena at interfaces between nanometer-sized electrodes and electrolytes. The aim is to provide a view on how the dimension comparability of nanoelectrodes with the electric double layer and the effective electron-tunnelling distance may raise distinct features in interfacial structure and reactivity. The strong coupling between the electrostatic field, the concentration field and the dielectric field of solvent at nanoelectrode/electrolyte interfaces is highlighted. The effects of this coupling on the voltammetric responses of nanoelectrodes are evaluated. Electron transfer kinetics at the nanoelectrode/electrolyte interface is discussed by emphasizing the inappropriateness of the Butler–Volmer (BV) and classic Marcus–Hush (MH) theories at potentials largely departing from the formal potential of the redox moieties and the importance of the long-distance electron tunnelling. The conditions for using the mathematically more straightforward BV and classic MH formalisms as an alternative to the physically more realistic but mathematically unfriendly Marcus–Hush–Chidsey model are analysed.
https://doi.org/10.1039/C3CP53773K