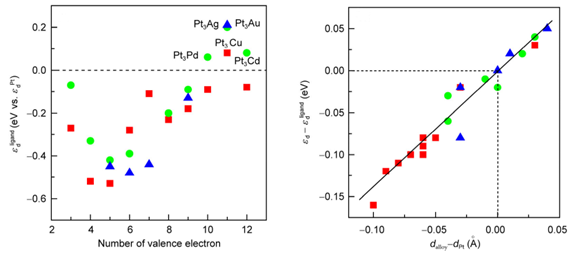
The stability and oxygen reduction reaction (ORR) activity of the Pt-segregated surface in various Pt-M alloys (M: transition metals) are investigated through systematic DFT calculations on the thermodynamic (alloy formation energy and Pt surface segregation energy), surface chemical property (oxygen binding energy) and electronic (d-band center) properties. Factors affecting these properties, such as the atomic radii and surface energy of M and the electronic ligand interaction between Pt and M are analyzed as a function of outmost d electron numbers of M. It is shown that the electronic ligand interaction plays determining role in the alloy formation energy of various Pt-M alloys; the formation of Pt-segregated surface in Pt-M alloys is favored when alloying metals have higher surface energy and smaller radii than Pt; the oxygen binding energy on the Pt-segregated surface in Pt-M alloys varies approximately linearly with the d-band center of surface Pt atoms; the lattice strain and electronic ligand effects are simply additive in Pt-M alloys; the stain effect in Pt-M alloys nearly linearly affects the d-band center of the Pt-segregated surface in Pt-M alloys; transition metals with less than 10 d electrons mostly exhibit electron ligand effects which result in downshift of the d-band center of the segregated surface Pt atoms, while those with ten d electrons exhibit electron ligand effect upshifting the d-band center of the segregated Pt atoms.
https://doi.org/10.1007/s11426-015-5324-y