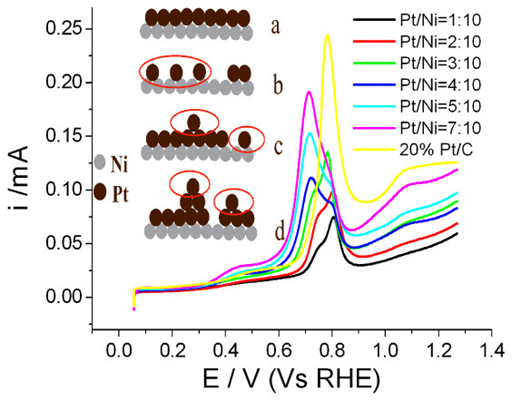
The electrochemical oxidation processes of CO and H2 have been used as probes to assess the surface states of carbon-supported Ni@Pt core-shell catalysts (Ni@Pt/C) with different Pt coverages in electrochemical environments. The CO-stripping cyclic voltammograms (CVs) of Ni@Pt/C catalysts present split double peaks representing two different states of Pt atoms on Ni-core particles. The current peaks in the CO-stripping CVs of Ni@Pt/C catalysts under relatively more positive potentials are consistent with that of Pt/C (a single peak), which indicates that some of the Pt atoms on Ni-core particles behave like pure Pt as isolated Pt atoms. The shoulder peaks shift toward more negative potentials, which may be because these Pt atoms on Ni-core particles assemble Pt–Pt bonds, resulting in Pt lattice constriction. The more positive current peaks in the CO-stripping CVs of Ni@Pt/C catalysts are predominant with lower Pt coverage, and with increasing Pt surface coverage, the more negative current peaks become prominent, which illustrates the integrity of Pt–Pt ensembles with neighboring Pt atoms. This phenomenon can be demonstrated by the schematic diagram of changes in the morphologies of Ni@Pt/C catalysts with increasing Pt coverage. HOR measurements further exhibit clearly regular patterns; here, the exchange current density (j0) values of Ni@Pt/C catalysts decrease with increasing Pt coverage and correspond to that of Pt/C at high Pt coverage. This result can be explained by the existence of nickel hydroxides and hydrogen spillover.
https://doi.org/10.1021/jp512283c