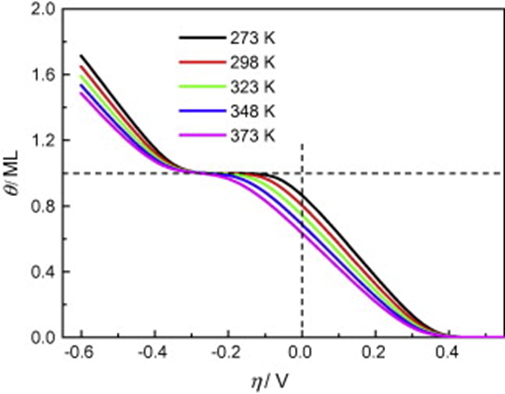
DFT calculations are combined with thermodynamic and kinetic modeling to study the effect of temperature on the electrochemical adsorption of hydrogen and the exchange current density (j0) of the hydrogen electrode reactions (HERs) on Pt(1 1 1). The nature of the underpotential and overpotential deposited hydrogen (UPD H and OPD H), the potential dependence of their coverage and the corresponding voltammetric curves for hydrogen adsorption are evaluated for temperatures from 273 K to 373 K. By introducing a coverage-dependent correction term in the free energy expression to account for the effect of the interaction between hydrogen ad-atoms on the configurational entropy, the DFT calculations give isotherms and voltammetric curves for hydrogen adsorption agreeing reasonably with those observed in experiments. It is shown that both the UPD and OPD H on Pt(1 1 1) surface could be the H atoms adsorbed at the 3-fold fcc hollow sites. A linear dependence of lnj0 on T−1 is found under temperatures from 273 K to 373 K, which, however, deviates from that predicted by the Arrhenius relation. The values of j0 and activation enthalpy for HERs on Pt(1 1 1) surface are estimated.
https://doi.org/10.1016/j.jelechem.2012.08.009