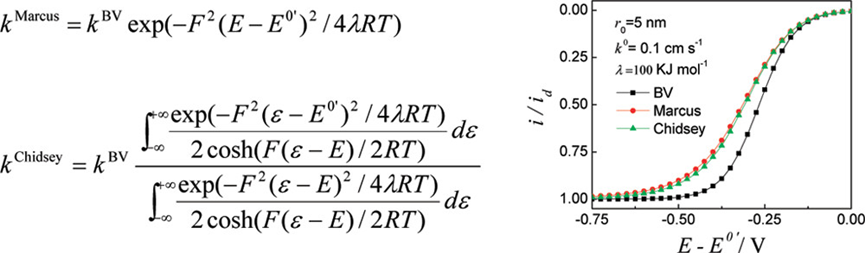Voltammetric responses of nanometer-sized electrodes are investigated theoretically by emphasizing the inappropriateness of the Butler-Volmer (BV) and Marcus theories in describing the heterogeneous electron transfer (ET) kinetics at potentials largely departing from the formal potential of the redox moieties and the importance of the distance-dependent electronic coupling at electrodes having sizes comparable to the effective electron-tunneling distances. It is shown that, for ET reactions with standard rate constant (k0) near 0.1 cm/s, the BV theory could predict voltammetric responses visibly deviated from that expected by the more realistic Chidsey model (Science1991, 215, 919–922), as the electrode radii (r0) are smaller than 50 nm. For ET reactions with k0 around 1.0 cm/s, this occurs as r0 approaches 10 nm. Except for ET reactions involving redox moieties with reorganization energy lower than 50 KJ/mol at electrodes smaller than 5 nm in r0, the Marcus formalism for the heterogeneous ET kinetics could predict voltammetric responses very similar to that given by the Chidsey model till the limiting current occurs. According to the voltammetric responses predicted by different ET theories, the reliability of the BV-based voltammetric analysis at nanoelectrodes in estimating the heterogeneous ET kinetics is discussed. The effect of the distance-dependent electronic coupling on the ET kinetics at spherical nanoelectrodes is modeled. It is shown that the standard rate constant will no longer be constant for an ET reaction on electrodes of certain materials as r0 goes below 10 nm, but decreases with decreasing the electrode size. However, the visible deviation from the constant value observed at large electrodes may occur only at electrodes smaller than 5 nm in radii, at which the uncertainties in the electrode shape and size could hinder the observation of any intrinsic electrode size effects.
https://pubs.acs.org/doi/full/10.1021/jp300696u