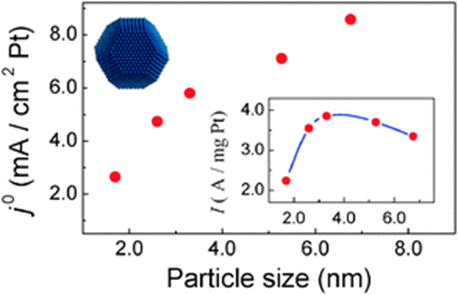
By using a catalyst-lean thin-film RDE method, the fast kinetics of the hydrogen oxidation reaction (HOR) on highly dispersed Pt nanoparticle electrocatalysts can be determined, free from the interference of the mass transport of H2 molecules in solution. Measurements with carbon-supported Pt nanoparticles of different sizes thus allow revealing the particle size effect of Pt for the HOR. It is shown that there is a “negative” particle size effect of Pt on the kinetics of HOR, i.e., the exchange current density j0 decreases with the increased dispersion (i.e. decreased mean particle size). A maximum mass activity of Pt for the HOR is found at particle sizes of 3–3.5 nm. The observed particle size effect is interpreted in terms of the size dependent distribution of surface atoms on the facets and edges, which is implied by the voltammetric responses of Pt/C catalysts with differently sized Pt particles. The accompanied decrease in the HOR activity with the increase in the edge atom fraction suggests that the edge atoms on the surface of Pt nanoparticles are less active for the HOR than those on the facets.
https://doi.org/10.1039/C2CP22761D