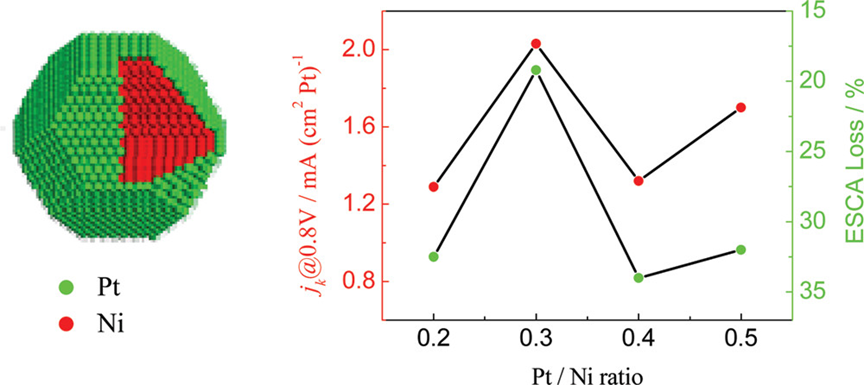
Core–shell nanoparticulate catalysts with a nonprecious metal core and a thin precious metal shell not only save precious metals but also could enhance the catalytic performance of precious metals through properly tuned strain and ligand effects. In this study, we show that, in addition to the nature and composition of core metals, the electrocatalytic properties of the precious metal shell can be tuned by varying its surface coverage. Carbon-supported Ni–Pt core–shell nanoparticle catalysts (Ni–Pt/C) with a series of Pt coverages are synthesized by depositing different amounts of Pt on Ni nanoparticles ca. 5 nm in size. It is found that Pt approximately forms an extended layer on Ni particles as its coverage does not exceed that required for a monolayer formation. The electrocatalytic properties of the Ni–Pt/C catalysts, such as the stripping potential for the oxygenated adsorbates, the activity for the oxygen reduction reaction (OPR), and the electrochemical stability under continuous potential cycling, exhibit a volcano type of dependence on Pt coverage, with the apex occurring near the monolayer. This suggests that core–shell nanoparticles with a monolayer Pt shell would be active and durable catalysts for the ORR and that extra Pt benefits neither the ORR activity nor the durability of the core–shell structured electrocatalysts.
https://pubs.acs.org/doi/full/10.1021/jp207828n?src=recsys