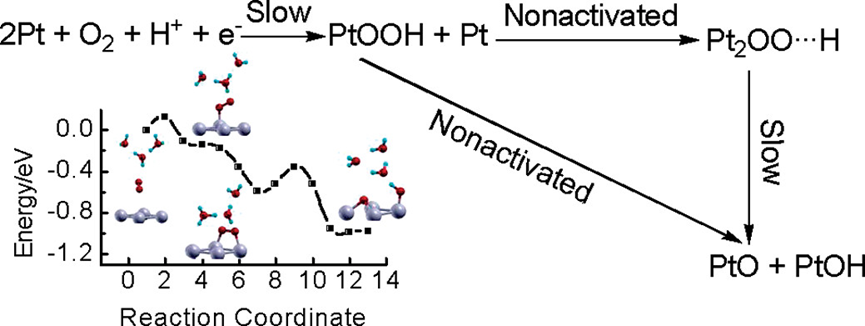
The adsorption and dissociation of O2 on the Pt(111) surface in both the absence and the presence of the hydrated proton were investigated using ab initio DFT calculations to evaluate the role of the proton in the initial steps of the Pt-catalyzed oxygen reduction reaction (ORR) in acid solutions. The results from geometric optimization and electronic structure and minimum energy path calculations indicated that, although in both cases, a t-b-t configured chemisorption state serves as the most stable molecular precursor for the dissociation of O2, the formation of this precursor state and its dissociation are substantially altered in the presence of the hydrated proton. The interactions of O2 with the hydrated proton inhibit the formation of the t-b-t precursor state but facilitate its dissociation. In the presence of the hydrated proton, the t-b-t molecular chemisorption of O2 is preceded by a metastable end-on chemisorption state that is protonated while the t-b-t state itself is not protonated. That is, the chemisorption of O2 on Pt in acid solution may undergo a sequential protonation and deprotonation process. It is also shown that the transformation from the end-on state to the t-b-t state is nearly a nonactivated process with the reaction energy larger in amount than the activation energy required for the subsequent dissociation. The formation of the end-on state via a proton-coupled electron-transfer process is, therefore, identified as the rate-determining step in the adsorption and dissociation processes of O2 on the Pt(111) surface in acidic media. The present calculation results may provide a link between the long disputed Damjanovic’s view and the Yeager’s view on the mechanism of the initial steps in ORR.
https://pubs.acs.org/doi/10.1021/jp9059505