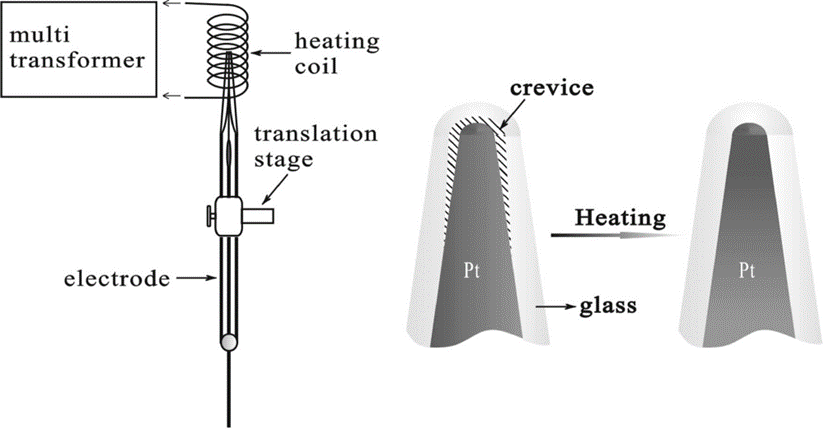
The voltammetric responses of Pt disk electrodes 5−50 nm in radii in the presence of excess inert electrolyte were investigated to verify the applicability of the conventional diffusion-based voltammetric theory to nanoscale electrochemical interfaces. A so-called “inverted heat-sealing” procedure was introduced in the electrode fabrication process to eliminate the possible tiny interstice between the glass sheath and electrode wire that could severely distort the voltammetric curves of nanometer-szied electrodes. Linear relations between the limiting currents (iL) and the concentrations of electroactive ions (ca) were found at electrodes as small as 5 nm, seemingly inferring that the classic voltammetric theory is applicable at such small electrodes. However, a delicate analysis on the dependences of iL on the electroactive size of electrode and the charge carried by the electroactive ions revealed that the iL ∼ ca linearity is altered from the predication of the conventional voltammetric theory as the size of electrode approaches nanometer scales (e. g., <10 nm). The altered iL ∼ ca linearity at nanoelectrodes is explainable in terms of size-induced merging of electric double layer (EDL) and concentration depletion layer (CDL) and is well-predictable from the previous dynamic double layer model for nanoelectrode based on Poisson−Nernst−Planck theory (J. Phys. Chem. B2006, 110, 3262). It is thus concluded that the enhanced EDL effects at nanoscale electrochemical interfaces do cause deviations from the predication of the conventional voltammetric theory, but the deviations are quantitatively small (e.g., within 20% even at electrodes of a few nanometers) and in most cases might be hardly distinguished with the experimental uncertainties.
https://pubs.acs.org/doi/10.1021/jp902311h