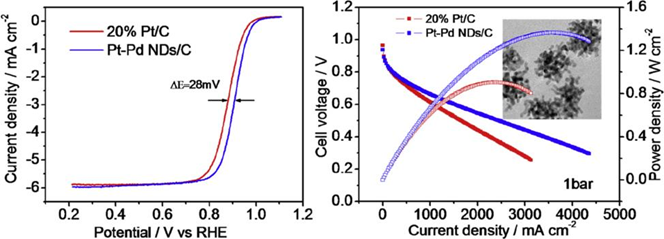
Platinum-palladium (Pt-Pd) bimetallic alloys have shown prospect as electrocatalyst for the oxygen reduction reaction (ORR) in the cathode of polymer-electrolyte-membrane (PEM) fuel cells. This article reports a facile solvothermal synthesis of Pt-Pd bimetallic nanodendrites (Pt-Pd NDs). The characterization with a variety of spectroscopic techniques indicates that the Pt-Pd NDs possess a three-dimensional (3-D) porous structure consisting of interconnected branches of highly alloyed Pt-Pd nanorods (NR). The measurements using rotating disk electrode in electrolyte solution show that the catalyst of Pt-Pd NDs supported on carbon (Pt-Pd NDs/C) possesses a Pt mass activity for ORR that is more than 3 times higher than that of the state-of-the-art Pt/C catalyst, as well as the significantly improved stability due to the branched porous structure. The measurements using membrane-electrode-assembly (MEA) in a single PEM fuel cell indicate the 3-D interconnected dendrite structures make the Pt-Pd NDs/C catalyst significantly advantageous over the nanoparticle Pt/C catalyst in reducing the mass transport and ohmic polarization which would become significant at high current density in MEA.
https://doi.org/10.1016/j.ijhydene.2017.08.162