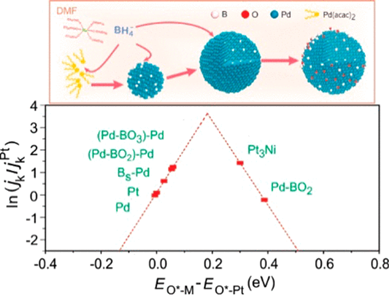
Boron doping can boost the catalytic activity of palladium for diverse reactions. Precise control of the doping content is crucial but remains difficult in current synthesis, which generally involves the use of instable and costly borane-organic compounds. Herein, by taking advantage of the relatively strong solvation of N, N-dimethylformamide (DMF) to Na+ and the increased stability BH4 − in DMF, we synthesize B-Pd interstitial nanocrystals in DMF, with NaBH4 acting as a reductant and boron source. The boron content, which can be readily tuned by changing the reaction time and NaBH4 concentration, can reach up to 20 at. %. Such a high boron doping results in a great beneficial effect on the catalytic capability of Pd toward the oxygen reduction reaction (ORR). The synthesized B-Pd nanoalloy exhibits a mass and specific activity for ORR that are, respectively, ca. 14 and 14.6 times higher than those of the state-of-the-art commercial Pt catalyst in alkaline solution. Density functional theory (DFT) calculations reveal three types of surface sites that are responsible for the enhanced activity, namely, Pd-BO2 assemblies, Pd atoms neighbored by the assemblies, and the Pd atoms modified with subsurface B atoms. The Pd-BO2 assembly has a Pt-like activity, while the neighboring Pd-BO2 assembly and subsurface B modified Pd atoms could catalyze ORR much more efficiently than Pt. The facile and controllable boron doping in palladium should strengthen the power of Pd-based catalysts and, therefore, provides great prospects for their widespread application.
https://doi.org/10.1021/acs.chemmater.7b03732