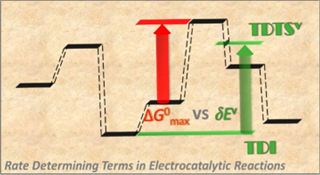
This work presents a new theoretical framework to construct volcano relations (VRs) that are capable of predicting and understanding the activity of top electrocatalysts. We propose a thermodynamic form of the energetic span (δE) as a rate-determining term for electrocatalytic reactions, which allows the essential factors such as the coverage of the stable intermediates and the energetics of the most demanding steps to be included in the rate equations and therefore overcomes the weakness of the current electrocatalytic VRs derived from kinetic models using the maximum standard free energy (ΔGmax°) of elementary reaction steps as rate-determining terms. The ΔGmax°-based VRs are shown to be applicable only at large overpotentials, where the intermediates involved in δE and ΔGmax° converge. At small overpotentials, where the excellent catalysts function, the ΔGmax°-based VRs may give improper predictions by missing the effects of the surface phases of stable adsorbates and their evolution with the potential. As well as revealing new features of electrocatalytic VRs and reasonably explaining some recent experimental results on efficient electrocatalysts for the hydrogen and oxygen electrode reactions, the δE-based rate models provide rich information about the catalytic mechanism and kinetics, e.g., the evolution of surface phases of stable intermediates, rate-determining transition states, and Tafel slopes.
https://pubs.acs.org/doi/full/10.1021/acscatal.8b03008