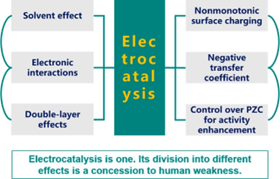
Electrocatalysis has been advanced to a point that understanding how noncovalent interactions interplay with covalent interactions becomes essential to precision design of efficient electrocatalysts. However, a conceptual framework for facilitating such understandings is yet missing; existing theories of electrocatalysis usually neglect noncovalent interactions. To fill in this gap, this study presents a refined theory of electrocatalysis by adding a noncovalent term that is self-consistently derived from a mean-field electrical double-layer (EDL) model into the model Hamiltonian, in addition to the electronic interaction term described by the Anderson–Newns model and the solvent term inspired by the Marcus theory. Applying the Green function technique allows us to portray the potential energy surface, to pinpoint multiple states along the reaction pathway and further to determine the reaction free energy and activation energies. Multifaceted interplay between covalent and noncovalent interactions is revealed. On the one hand, chemisorption-induced surface dipoles modify the work function and electrostatic properties of the metal, leading to nonmonotonicity in the surface charging relation and then the activation energy profile. As an immediate consequence, the sum of anodic and cathodic transfer coefficient is less than unity and the cathodic transfer coefficient can even be negative. On the other hand, EDL effects modulate covalent interactions, in return, via dictating the electrochemical potential of ions. The theory brings forth a new adsorption isotherm, which provides fundamental insights into the perplexing strong indirect adsorbate–adsorbate interactions, and furnishes a theoretical approach to analyze the electrosorption valency as well.
https://pubs.acs.org/doi/10.1021/acs.jpcc.8b07534