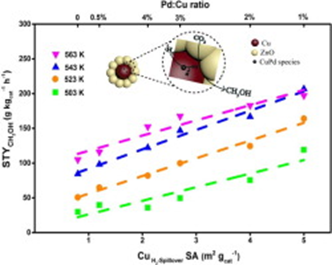
Surface modification with Pd is an effective way for improved activity in CO hydrogenation to methanol over commercial Cu-ZnO catalysts via a so-called hydrogen spillover mechanism. However, there still lacks a quantitative analysis of hydrogen spillover effect and the nature of active sites after Pd modification remains unclear. In this work, we prepared a series of Pd-doped Cu-ZnO catalysts (Pd-CZ-x) with tunable Pd loading by using a facile polyol reduction method for a deep study of the promotion effect of Pd. With the increase of Pd/Cu molar ratio (x) from 0 to 0.04, there emerges a volcano-shaped relationship between methanol space time yield (STY) and Pd loading. 1% Pd doping can increase the methanol STY by 2.5 times and the methanol turnover frequency (TOF) by 3.5 times at 543 K, when compared to undoped Cu-ZnO. Kinetic studies show the activation energy required for methanol synthesis is greatly reduced from 59 kJ mol-1 over Cu-ZnO to 31 kJ mol over Pd-CZ-0.01. Behind the volcano-shaped relationship is a balance between the hydrogen spillover effect of Pd and the reduced surface Cu area caused by Pd blocking. Chemisorption gives a quantitative analysis of an important type of activated Cu sites enabled by hydrogen spillover (Cu). Importantly, it is found that the methanol STY correlates linearly with Cu surface area, suggesting that the activated Cu sites with the assistance of ZnO are real active sites for methanol synthesis from CO hydrogenation.
https://doi.org/10.1016/j.jcat.2017.12.029