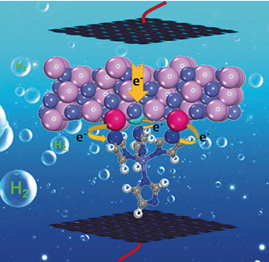
Although electrocatalysts based on transition metal phosphides (TMPs) with cationic/anionic doping have been widely studied for hydrogen evolution reaction (HER), the origin of performance enhancement still remains elusive mainly due to the random dispersion of dopants. Herein, we report a controllable partial phosphorization strategy to generate CoP species within the Co-based metal-organic framework (Co-MOF). Density functional theory calculations and experimental results reveal that the electron transfer from CoP to Co-MOF through N-P/N-Co bonds could lead to the optimized adsorption energy of H2O (ΔGurn:x-wiley:00448249:media:ange201901409:ange201901409-math-0001 ) and hydrogen (ΔGH*), which, together with the unique porous structure of Co-MOF, contributes to the remarkable HER performance with an overpotential of 49 mV at a current density of 10 mA cm−2 in 1 m phosphate buffer solution (PBS, pH 7.0). The excellent catalytic performance exceeds almost all the documented TMP-based and non-noble-metal-based electrocatalysts. In addition, the CoP/Co-MOF hybrid also displays Pt-like performance in 0.5 m H2SO4 and 1 m KOH, with the overpotentials of 27 and 34 mV, respectively, at a current density of 10 mA cm−2.
https://onlinelibrary.wiley.com/doi/full/10.1002/ange.201901409