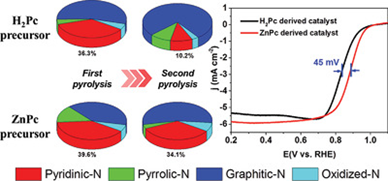
Nitrogen (N)-doped carbons are potential nonprecious metal catalysts to replace Pt for the oxygen reduction reaction (ORR). Pyridinic-N-C is believed to be the most active N group for catalyzing ORR. In this work, using zinc phthalocyanine as a precursor effectively overcomes the serious loss of pyridinic-N, which is commonly regarded as the biggest obstacle to catalytic performance enhancement upon adopting a second pyrolysis process, for the preparation of a 3D porous N-doped carbon framework (NDCF). The results show only ≈14% loss in pyridinic-N proportion in the Zn-containing sample during the second pyrolysis process. In comparison, a loss of ≈72% pyridinic-N occurs for the non-Zn counterpart. The high pyridinic-N proportion, the porous carbon framework produced upon NaCl removal, and the increased mesoporous defects in the second pyrolysis process make the as-prepared catalyst an excellent electrocatalyst for ORR, exhibiting a half-wave potential (E1/2 = 0.88 V) up to 33 mV superior to state-of-the-art Pt/C and high four-electron selectivity (n > 3.83) in alkaline solution, which is among the best ORR activities reported for N-doped carbon catalysts. Furthermore, only ≈18 mV degradation in E1/2 occurs after an 8000 cycles' accelerating stability test, manifesting the outstanding stability of the as-prepared catalyst.
https://onlinelibrary.wiley.com/doi/full/10.1002/smll.201805325