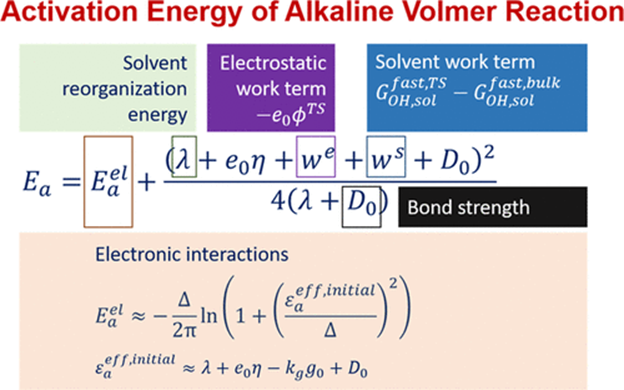
The sluggish kinetics of hydrogen evolution/oxidation reactions in alkaline media remains a technical barrier for alkaline membrane fuel cells and a scientific puzzle under heated discussion in fundamental electrocatalysis. Much attention has been centered around thermodynamic origins, whereas microscopic kinetics is less understood and a quantitative account of key factors is yet missing. To fill in this gap, a microscopic Hamiltonian model is developed for the alkaline Volmer step, an elementary step of hydrogen reactions, encompassing electronic interactions, bond breaking, solvent reorganization, and double-layer electrostatic effects. The model gives out a simple yet informative analytical formula for the activation barrier of the alkaline Volmer step, quantifying the contributions of various factors; roughly speaking, one quarter of the H-OH bond energy enters into the activation energy. This model elucidates that the larger activation energy seen at a more charged interface is not because it is more difficult to reorganize the solvents but rather because it consumes more work in bringing OH- to the double layer, namely, a larger work term. Previous strategies used to boost the activity of hydrogen reactions in alkaline media are rationalized in a coherent framework.
https://pubs.acs.org/doi/10.1021/acs.jpcc.9b03639