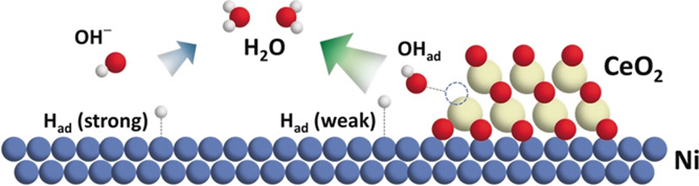
The search for highly efficient platinum group metal (PGM)-free electrocatalysts for the hydrogen oxidation reaction (HOR) in alkaline electrolytes remains a great challenge in the development of alkaline exchange membrane fuel cells (AEMFCs). Here we report the synthesis of an oxygen-vacancy-rich CeO2/Ni heterostructure and its remarkable HOR performance in alkaline media. Experimental results and density functional theory (DFT) calculations indicate the electron transfer between CeO2 and Ni could lead to thermoneutral adsorption free energies of H* (ΔGH*). This, together with the promoted OH* adsorption strength derived from the abundance of oxygen vacancies in the CeO2 species, contributes to the excellent HOR performance with the exchange current density and mass activity of 0.038 mA cmNi−2 and 12.28 mA mgNi−1, respectively. This presents a new benchmark for PGM-free alkaline HOR and opens a new avenue toward the rational design of high-performance PGM-free electrocatalysts for alkaline HOR.
https://doi.org/10.1002/anie.201908194