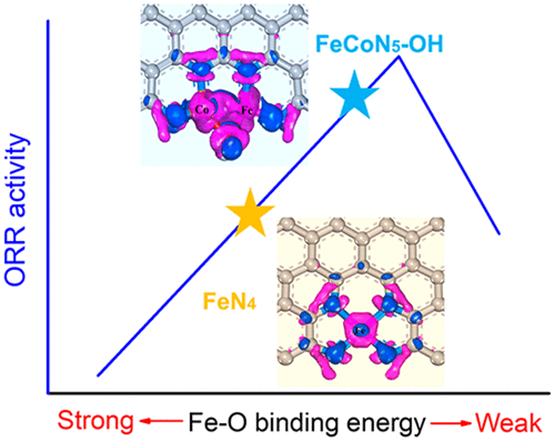
Great enthusiasm in single-atom catalysts (SACs) for the oxygen reduction reaction (ORR) has been aroused by the discovery of M–NX as a promising ORR catalysis center. However, the performance of SACs lags far behind that of state-of-the-art Pt due to the unsatisfactory adsorption–desorption behaviors of the reported catalytic centers. To address this issue, rational manipulation of the active site configuration toward a well-managed energy level and geometric structure is urgently desired, yet still remains a challenge. Herein, we report a novel strategy to accomplish this task through the construction of an Fe–Co dual-atom centered site. A spontaneously absorbed electron-withdrawing OH ligand was proposed to act proactively as an energy level modifier to empower easy intermediate desorption, while the triangular Fe–Co–OH coordination facilitates O–O bond scission. Benefiting from these attributes, the as-constructed FeCoN5–OH site enables an ORR onset potential and half-wave potential of up to 1.02 and 0.86 V (vs RHE), respectively, with an intrinsic activity over 20 times higher than the single-atom FeN4 site. Our finding not only opens up a novel strategy to tailor the electronic structure of an atomic site toward boosted activity but also provides new insights into the fundamental understanding of diatomic sites for ORR electrocatalysis.
https://doi.org/10.1021/jacs.9b08362