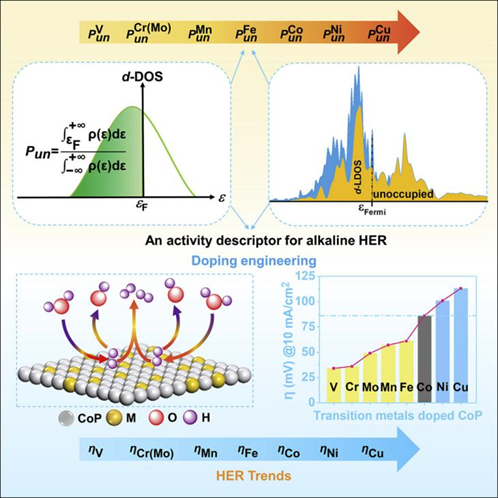
Understanding the mechanism of the alkaline hydrogen evolution reaction (HER) and finding a design principle to enhance the catalytic performance of electrocatalysts are of great significance in paving the way of alkaline water electrolysis. Here, taking cobalt phosphide (CoP) as a model material, we establish the alkaline HER activity trends as a function of a fundamental property, the proportion of the unoccupied 3d orbital (Pun). By doping CoP with a range of 3d and 4d transition metals with different Pun, we show that the intrinsic alkaline HER kinetics increases with Pun. DFT calculations indicate that the transition metals with higher Pun not only act as the oxophilic sites for enhanced water activation but also modulate the electronic structure of CoP to endow an optimized H adsorption (ΔG∗H). Moreover, by further comparing the correlation between water adsorption and dissociation or ΔG∗H and experimental alkaline HER activities, we find that water dissociation is the rate-determining step for alkaline HER.
https://doi.org/10.1016/j.xcrp.2020.100136