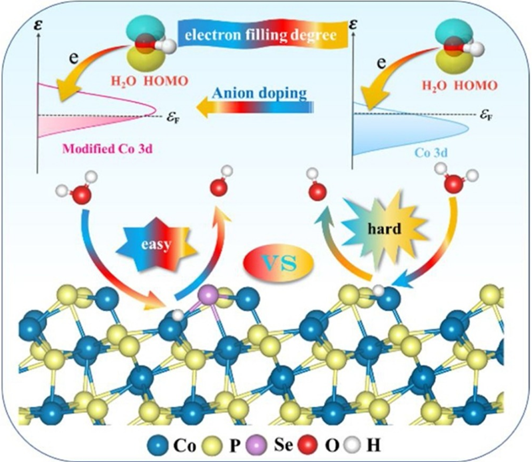
Understanding the origins of the sluggish kinetics and finding a descriptor to predict the alkaline hydrogen evolution reaction (HER) activity are crucial for the rational design of advanced transition metal-based electrocatalysts. Here, taking CoP as a model, we develop a strategy at the atomic level to boost the alkaline HER performance through tailoring the 3d-orbital electron filling degree of Co center by anions engineering. We find the water dissociation step rather than hydrogen adsorption-desorption is the root of sluggish alkaline HER kinetics. As expected, the obtained Se-doped CoP (Se-CoP) delivers remarkable HER performance with the overpotential of 41 mV at 10 mA cm−2 and TOF value of 0.158 s−1 at 200 mV in alkaline electrolyte. Moreover, we have also proved that this design principle can be applied to other anions (O and S) doped CoP, providing a new avenue for rational design of transition metal-based electrocatalysts towards alkaline HER.
https://doi.org/10.1016/j.apcatb.2020.119718