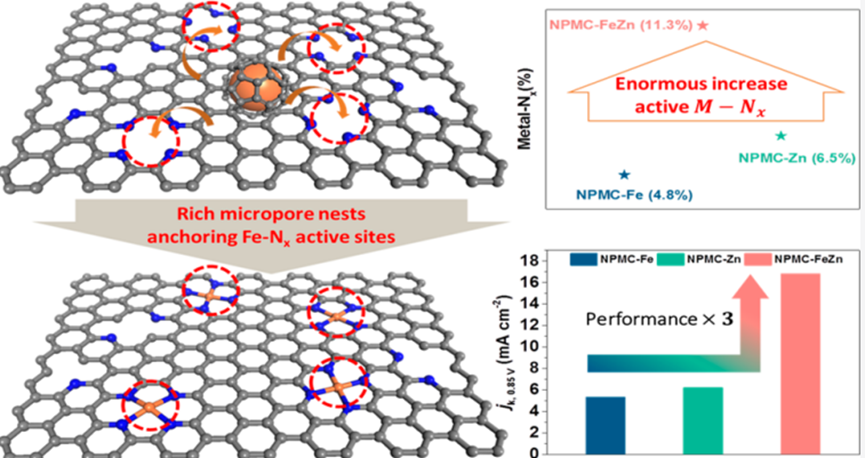
Iron and nitrogen codoped carbons (Fe-Nx-C) are potential nonprecious metal catalysts to substitute Pt for oxygen reduction reaction (ORR). The essential goal for Fe-Nx-C catalysts is to obtain high-density and well-dispersed Fe-Nx active sites. Herein, we demonstrate that this can be realized by a NaCl crystallites-templated copyrolysis of iron phthalocyanine (FePc) and zinc phthalocyanine (ZnPc), in which Zn vitally induces rich micropores and stabilizes the edge-doped N atoms, thus providing effective footholds for the formation of well-dispersed high-density Fe-Nx coordination sites. The produced Fe, Zn-containing nitrogen-doped carbon framework (NPMC-FeZn) exhibits superior ORR activity to NPMC-Fe and NPMC-Zn formed by pyrolysis of FePc and ZnPc individually. In NPMC-Fe, large amounts of Fe are in the form of Fe-oxides nanoparticles other than Fe-Nx coordination sites, while NPMC-Zn contains mainly Zn-Nx sites that are relatively less active for ORR. Specifically, NPMC-FeZn exhibits half-wave potentials (E1/2) of 0.90 V in 0.1 M KOH (up to 53 mV superior to the Pt/C) as well as the high four-electron selectivity. Furthermore, a minimal ca. 30 mV decline in E1/2 occurs after 5000 cycles in O2-saturated 0.1 M KOH, manifesting the outstanding electrochemical stability of NPMC-FeZn.
https://doi.org/10.1021/acs.jpcc.1c08470