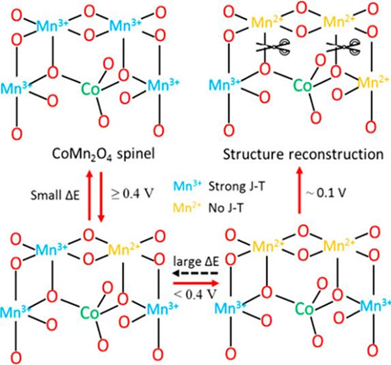
The Co-Mn spinel oxides have attracted much research attention as a class of low-cost electrocatalysts toward the oxygen reduction reaction (ORR) owing to their promising performance in anion-exchange membrane fuel cells (AEMFCs). In this work, the nature of the active sites in a representative CoMn2O4 catalyst was investigated with the assistance of in situ X-ray absorption spectroscopy (XAS) within the ORR-relevant potential range. Our work revealed that the superior activity of the Co–Mn spinel oxides relates to the Mn2+/Mn3+ redox transition. The Mn2+/Mn3+-associated activity is largely affected by the operating potential window, i.e., an activity loss would be observed for Co–Mn spinel oxides operated at potential lower than 0.4 V (vs RHE). It is proposed that this irreversible activity decay is caused by the irreversible change of the Jahn-Teller (J-T) distortion during the Mn2+/Mn3+ transition.
http://doi-org-s.vpn.whu.edu.cn:8118/10.1021/acs.jpcc.1c00104