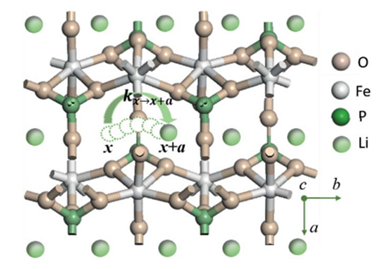
The discrepancy between the trend in the diffusion coefficient of a lithium ion (DLi+) and that in the activation energy of ion hopping signals hidden factors determining ion transport kinetics in layered olivine phosphate materials (LiMPO4). Combining density functional theory (DFT) calculations and the Landau–Zener electron transfer theory, we unravel this hidden factor to be the electronic coupling between redox centers of the host materials. The ion transport process in LiMPO4 is newly described as an ion-coupled electron transfer (ET) reaction, where the electronic coupling effect on DLi+ is considered by incorporating the electronic transmission coefficient into the rate constant of the transfer reaction. The new model and DFT calculation results rationalize experimental values of DLi+ for various LiMPO4 (M = Fe, Mn, Co, Ni) materials, which cannot be understood solely by the calculated activation barrier of ion hopping. Interestingly, the electronic coupling between host redox centers is found to play an essential role. Particularly, the sluggish ion mobility in LiFePO4 is due to a very weak electronic coupling. The obtained insights imply that one can improve the rate performance of intercalation materials for metal-ion batteries through modifying the electronic coupling between redox centers of host materials.
https://pubs.rsc.org/en/content/articlehtml/2022/sc/d1sc05402c