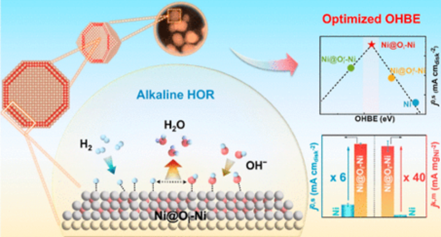
Precisely tailoring the electronic structures of electrocatalysts to achieve an optimum hydroxide binding energy (OHBE) is vital to the alkaline hydrogen oxidation reaction (HOR). As a promising alternative to the Pt-group metals, considerable efforts have been devoted to exploring highly efficient Ni-based catalysts for alkaline HOR. However, their performances still lack practical competitiveness. Herein, based on insights from the molecular orbital theory and the Hammer–Nørskov d-band model, we propose an ingenious surface oxygen insertion strategy to precisely tailor the electronic structures of Ni electrocatalysts, simultaneously increasing the degree of energy-level alignment between the adsorbed hydroxide (*OH) states and surface Ni d-band and decreasing the degree of anti-bonding filling, which leads to an optimal OHBE. Through the pyrolysis procedure mediated by a metal–organic framework at a low temperature under a reducing atmosphere, the obtained oxygen-inserted two atomic-layer Ni shell-modified Ni metal core nanoparticle (Ni@Oi-Ni) exhibits a remarkable alkaline HOR performance with a record mass activity of 85.63 mA mg–1, which is 40-fold higher than that of the freshly synthesized Ni catalyst. Combining CO stripping experiments with ab initio calculations, we further reveal a linear relationship between the OHBE and the content of inserted oxygen, which thus results in a volcano-type correlation between the OH binding strength and alkaline HOR activity. This work indicates that the oxygen insertion into the top-surface layers is an efficient strategy to regulate the coordination environment and electronic structure of Ni catalysts and identifies the dominate role of OH binding strength in alkaline HOR.
https://doi.org/10.1021/jacs.2c01448