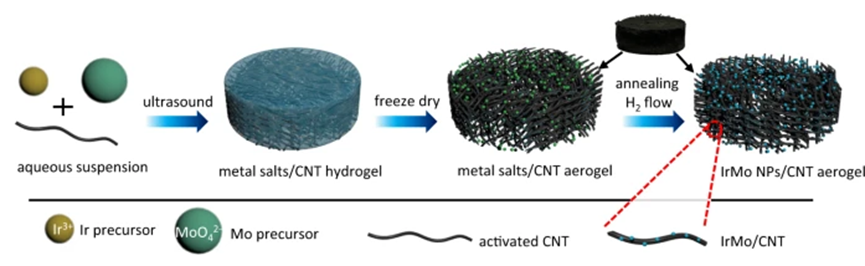
The sluggish kinetics of the hydrogen evolution reaction in base has resulted in large activity gap between acidic and alkaline electrolytes. Here, we present an intermetallic IrMo electrocatalyst supported on carbon nanotubes that exhibits a specific activity of 0.95 mA cm−2 at the overpotential of 15 mV, which is 14.4 and 9.5 times of those for Ir/C and Pt/C, respectively. More importantly, its activities in base are fairly close to that in acidic electrolyte and the activity gap between acidic and alkaline media is only one fourth of that for Ir/C. DFT calculations reveal that the stably-adsorbed OH spectator at Mo site of IrMo can stabilize the water dissociation product, resulting in a thermodynamically favorable water dissociation process. Beyond offering an advanced electrocatalyst, this work provides a guidance to rationally design the desirable HER electrocatalysts for alkaline water splitting by the stably-adsorbed OH spectator.
https://doi.org/10.1038/s41467-022-33216-w