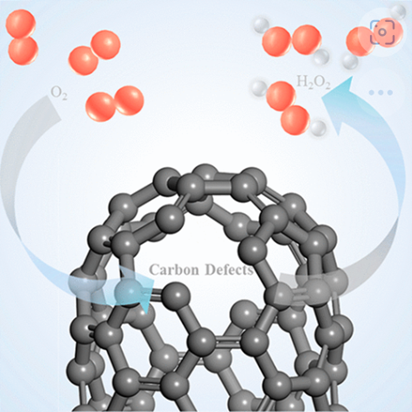
Carbon materials have manifested promising potential in electrochemical reduction of O2 to H2O2. The oxygen functional groups have been identified as the catalytic sites. However, the intrinsic carbon defects abundant in carbon materials have often been neglected. Herein, a three-dimensional carbon framework with abundant intrinsic defects and oxygen functional groups (the oxygen content and chemical states of oxygen are comparable to those of commercial carbon black) was introduced and exhibited outstanding catalytic activity and selectivity toward H2O2 electrosynthesis. Through a combination of in situ Raman spectroscopy and density functional theory calculations, the intrinsic carbon defects, such as zigzag edge and zigzag pentagon sites with optimal binding energy for OOH, were also determined to be active sites. It was further revealed that intrinsic carbon defects with large negative charge density and asymmetric spin density may have high activity toward H2O2 production.
https://doi.org/10.1021/acs.jpclett.2c02684